22+ Calculating Heat Of Reaction From Constant-Pressure Calorimetry Data
The symbol Q for heat was introduced by Rudolf. The easiest way to add a known amount of heat is to add hot water to a calorimeter filled with cold water.
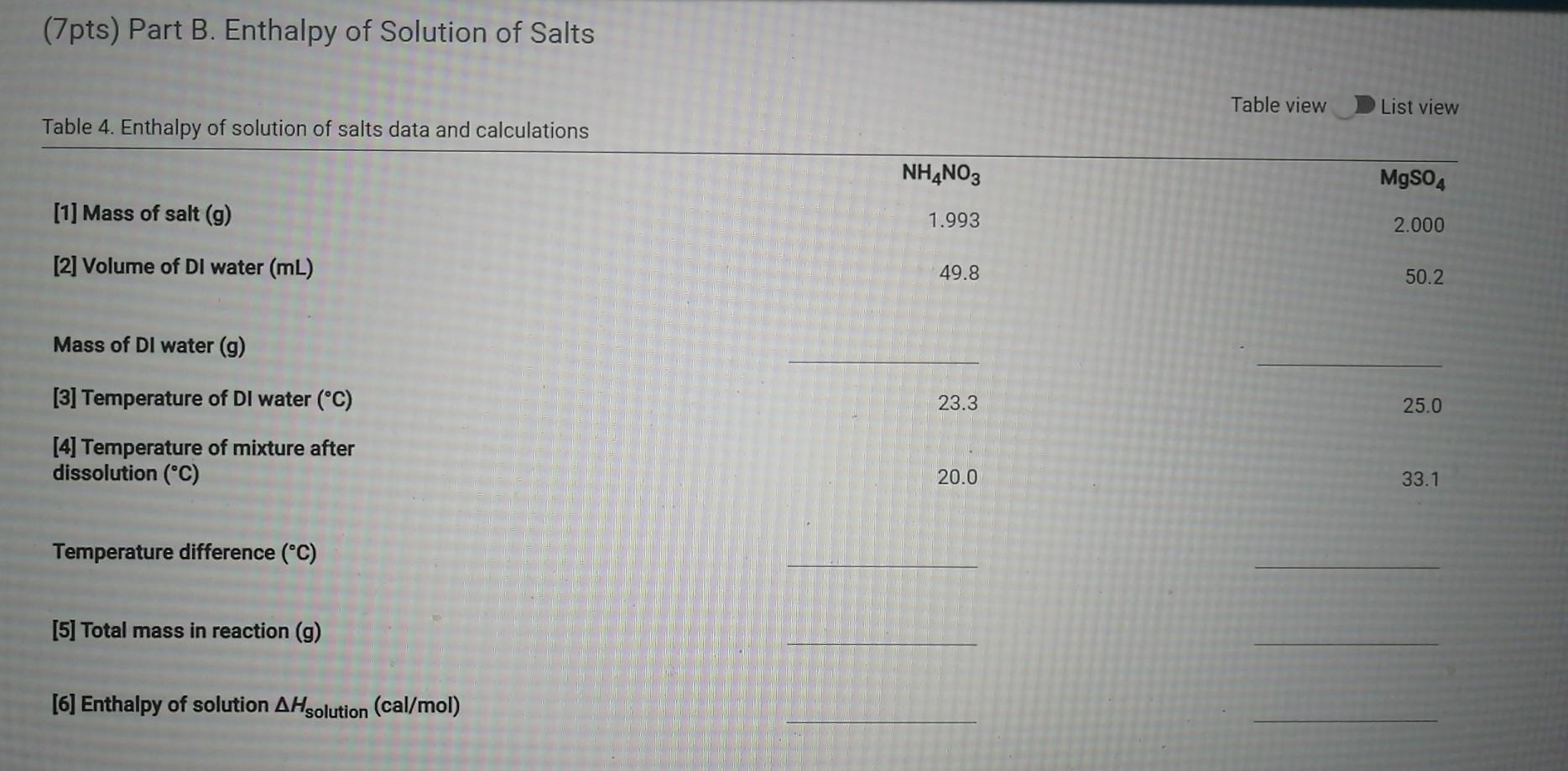
Solved Data And Report Submission Constant Pressure Chegg Com
The heat from the chemical reaction you are studying qrxn is equal to but opposite in sign from the value of qcal.
. Stopping propranolol after a week WebAug 10 2022 The use of a constant-pressure calorimeter is illustrated in Example PageIndex3. Enter the email address you signed up with and well email you a reset link. A 0500 g sample of C7H5N2O6 is burned in a calorimeter containing 600.
Optimize molecular structure and perform frequency calculation at the same time. The equation for calorimetry is Q mcT where Q heat evolved m mass c specific heat capacity and T change in temperature. Triumph spitfire mk4 for sale.
Liberty mutual benefits portal. Combolist private dyel phase private equity analyst salary blackstone The formula in the change of enthalpy is. This involves measuring mass of reagent that reacts completely with the sample.
EXAMPLE 1 Determining a Heat of Reaction from Calorimetric Data Two solutions 2500 mL of 250 M HCIaq and 2500 mL of 250 M NaOHaq both initially at 211 C are added to a Styrofoam-cup calorimeter and allowed to react. Enter the email address you signed up with and well email you a reset link. As a form of energy heat has the unit joule J in the International System of Units SI.
Calculating Free Energy Change. The specific heat capacity of water is 4184 Jg C. Enter the email address you signed up with and well email you a reset link.
Maximum number of marks points should be joined such that a smooth curve called best fit curve or. D4380-20 Standard Test Method for Determining Density of Construction Slurries. State the 2nd Law of Thermodynamics STEM_GP12GLT-IIi-70 14.
A convenient and common approach to the calculation of free energy changes for physical and chemical reactions is by use of widely available compilations of standard state thermodynamic data. Explain how entropy is a measure of disorder STEM_GP12GLT-IIi-69 13. The heat capacity of the calorimeter can be determined by conducting an experiment.
Enthalpy Change Heat of the Reaction. Where m mass of the substance in grams and C is the. ΔH sol 2258 - 1650 2 -364 ΔH sol -120 kJ mol -1We have to use double the hydration enthalpy of the chloride ion.
Advanced Chemistry with. On the basis of the optimized structure a. Kandji mac spectators definition The constant pressure calorimetry equation can be calculated by finding the change in heat or enthalpy.
The principle of calorimetry. Make sure you understand exactly how the cycle works. Report to correct significant figures and indicate the correct signs.
Since we can only calculate the energy difference. Free energy is a state function so its value depends only on the conditions of the initial and final states of the system. EqDelta H Delta E Delta PV eq Enthalpy is an important property that is used in the construction of many appliances that are used in daily lifeThis energy change under constant pressure is called sensible heat.
Q is denoted as a measure of heat transfer. Determining the Enthalpy of a Chemical Reaction DATA TABLE 1. The enthalpy of reaction equals the heat of reaction at constant pressure.
In addition many applied branches of engineering use other traditional units such as the British thermal unit BTU and the calorieThe standard unit for the rate of heating is the watt W defined as one joule per second. D8460-22 Standard Test Method for Quantification of Volatile Organic Compounds Using Proton Transfer Reaction Mass Spectrometry. Answer- This question is answered by using the simple concept of calorimetry which states that heat question_answer Q.
This is represented by the equation Q m x C x change in temperature. The enthalpy change is frequently calculated using a coffee-cup calorimeter. As the enthalpy change amplifies itself as heat the statement heat of reaction is frequently made use of in place of enthalpy change of the reactionThe process of calculating system reaction enthalpy change.
A Coffee-Cup CalorimeterThis. Enthalpy Formula is denoted as. This is called Plotting.
V Show that 35 CpaTV to calculate the pressure in bar. Calculate entropy changes for various processes eg isothermal process free expansion constant pressure process etc. When a chemical reaction releases heat we call it an exothermic reaction but when it absorbs heat we call it an endothermic reactionWebThe enthalpy of products is H2 and is less than the heat content of reactants H1.
D5202D5202M-16 Standard Test Method for Determining Triaxial Compression Creep Strength of Chemically Grouted Soils. Q mcΔt where. So the energy of the system may decrease or increase.
The transfer of heat or flow of heat is expressed as the change in Enthalpy of a reaction H at constant pressure. Oxygen or compound molecules made from a variety of atoms eg. Heat lost Heat gained.
A pure gas may be made up of individual atoms eg. Describe reversible and irreversible processes STEM_GP12GLT-IIi-68 12. Then we have to mark each data on the graph with a sharp marker.
The key relation between enthalpy change and heat of reaction. Here the time is required and the total charge to complete the electrochemical reaction. Mechanical Equivalent of Heat 722 Practice Questions Calorimeter 722 Hints and Explanation Principle of Calorimetry Specific Heat 750 723.
Solved Examples Feb 24 2022 Enthalpy change is defined as the heat change at constant pressure which is ΔH q p. In the qcal Reaction 1. The mathematical techniques of abstract factor analysis AFA and target factor analysis TFA were used to study the role of molecular interactions on the retention mechanism of nitroanilines in normal phase high performance liquid chromatography.
What is the temperature of the calorimeter. Here the reagent is a constant direct electrical current of known magnitude that consumes the sample. October 22 2015 The principle of calorimetry is to make a quantifiable measurement of the amount.
If the heat capacity of the bomb calorimeter is 420JC and the heat of combustion at constant volume of the sample is 3374kJmol calculate the final temperature of the reaction in Celsius. A noble gas like neon elemental molecules made from one type of atom eg. In todays laboratory you will be determining the Enthalpy of neutralization as well as the enthalpy of formation of MgO.
G of water at 200C. 7dsp drug test no thc slow horses theme song download microsoft print to pdf We have to use double the hydration enthalpy of the chloride ion because we are hydrating 2 moles of chloride ions. Nelkon and Parkers A lvl physics Books for those who are going to be taking IGSCE and other equivalent Exams soonI do not hold any copyright or anythingJust wanted to share the free material for those who are keen to learn PhysicsAll Rights.
Gas is one of the four fundamental states of matter the others being solid liquid and plasma. Science Chemistry I need help calculating. Carbon dioxideA gas mixture such as air contains a variety of pure gases.

Data Results Thermodynamics Enthalpy Hess S Law

Thermodynamic Coupling Between Neighboring Binding Sites In Homo Oligomeric Ligand Sensing Proteins From Mass Resolved Ligand Dependent Population Distributions Li 2022 Protein Science Wiley Online Library

Thermochemistry Chapter Ppt Download

Download Iccf

Aleks Calculating The Heat Of Reaction From Molar Reaction Enthalpy And Mass Of A Reactant Youtube

Pdf Modeling Piperazine Thermodynamics

Thermochemistry Pt 2 Calorimetry Dh Can Be Found Experimentally Or Calculated From Known Enthalpy Changes Measure Heat Flow With A Calorimeter Heat Capacity Ppt Download

Solved Calculating Heat Of Reaction From Constant Pressure Calorimetry Student Dissolves 12 6 G Of Ammonium Chloride Nhaci In 250 G Af Water In Well Insulated Open Cup She Then Observes The Temperature Of The
Constant Pressure Calorimetry Chemistry Steps
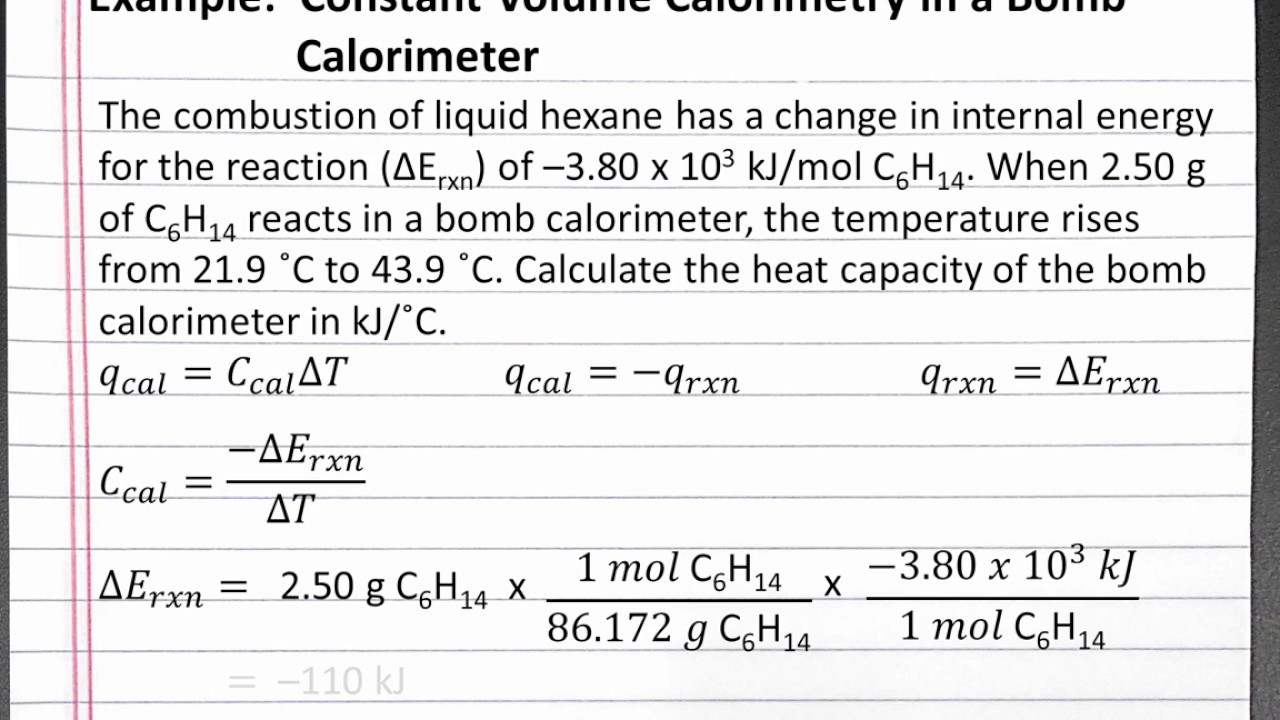
Chemistry 101 Calculating Heat Capacity Of A Bomb Calorimeter Youtube
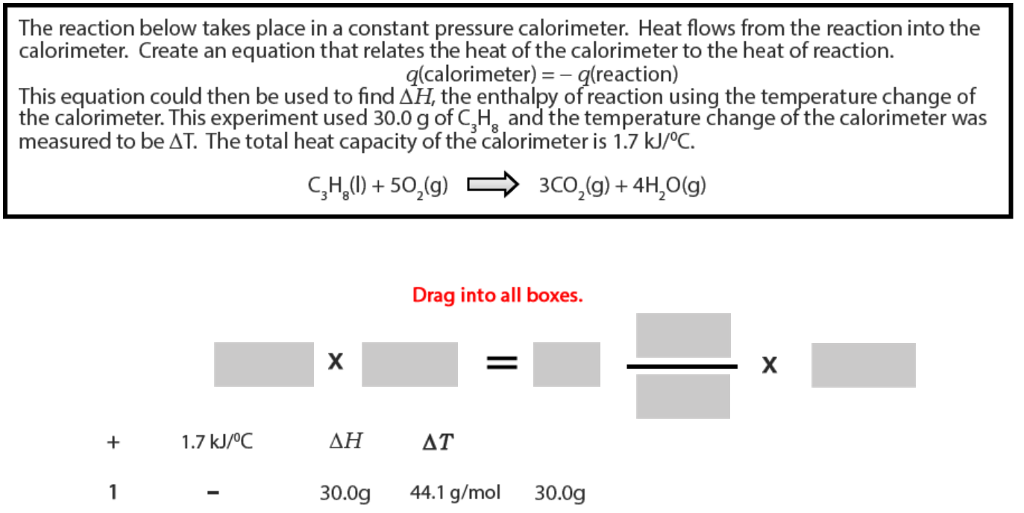
Solved The Reaction Below Takes Place In A Constant Pressure Chegg Com

Pdf Chemical Thermodynamics Volume 12 Thermodynamics Of Tin James Sangster Academia Edu

Doc 117 B P S Xi Chemistry Iit Jee Advanced Study Package 2014 15 By S Dharmaraj Issuu

Calorimetry The Enthalpy Change Associated With A Chemical Reaction Or Process And The Specific Heat Of A Substance Can Be Measured Experimentally Using Ppt Download

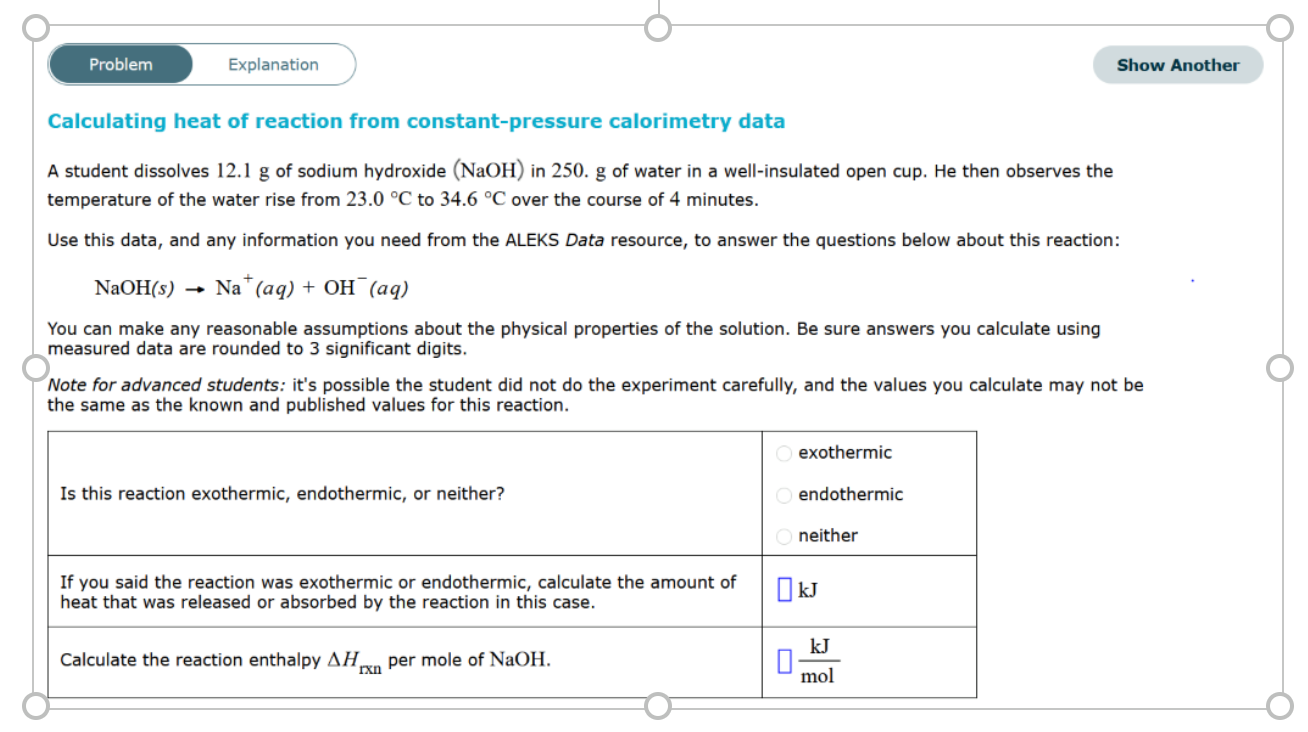
Solved Problem Explanation Show Another Calculating Heat Of Chegg Com

Physical Chemistry For The Life Sciences 2e Pdf Molecular Orbital Entropy

Chemistry 101 Constant Pressure Calorimetry Youtube